Written by J.A Dobado | Last Updated on April 22, 2024
Haloalkanes or alkyl halides are compounds derived from alkanes (CnHn) that have one or more halogen functional groups (F, Cl, Br, I) in the carbon chain.
The most characteristic reactions are listed below.
Reduction to alkanes
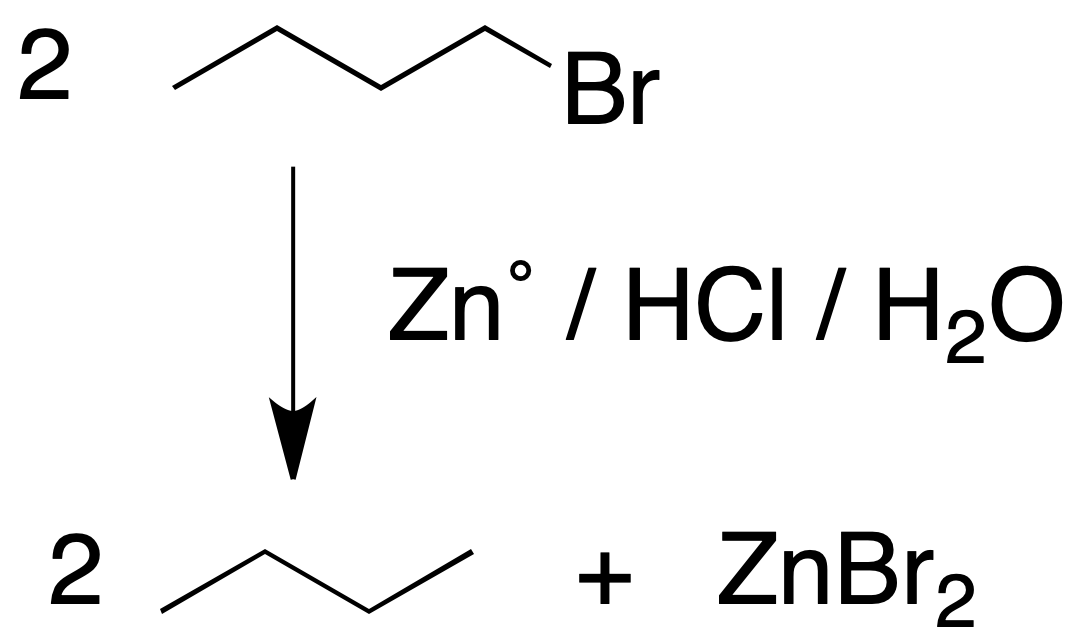
Reaction of alkyl halides with Zno in an acid medium
Treatment with metallic Zn with an aqueous acid of an alkyl halide leads to the formation of an alkane and the corresponding zinc halide. It is a general process for the substitution of a halogen atom by hydrogen.
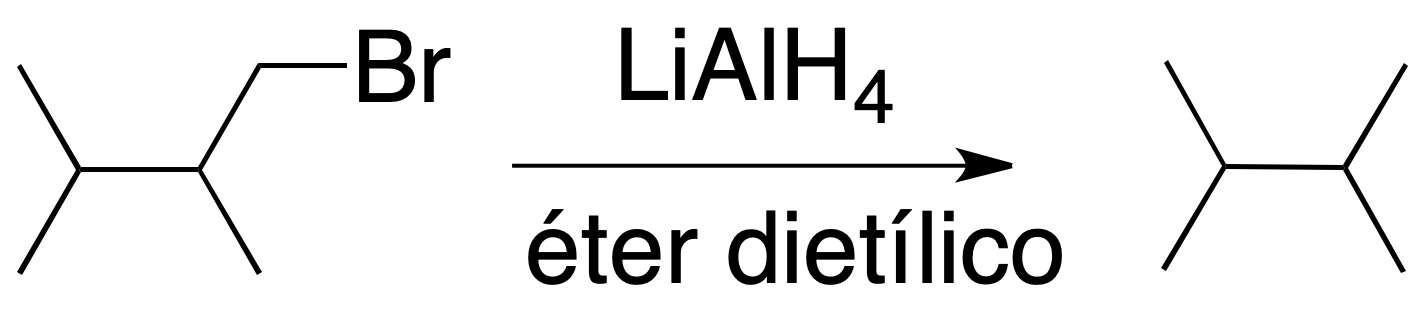
Reaction of alkyl halides with LiAlH4
Primary and secondary alkyl halides react with good yields with LiAlH4 in diethyl ether to give alkanes. The result is the substitution of a halogen atom for hydrogen.
LiAlH4 reacts violently with water and alcohols, being incompatible with a number of functional groups present in the molecule, for example:
| Functional group | Final product |
|---|---|
| Nitro-compound | Amine |
| Amide | |
| Azide | |
| Nitrile | |
| Aldehyde | Primary alcohol |
| Acyl chloride | |
| Carboxylic acid | |
| Ester | |
| Ketone | Secondary alcohol |
| Epoxide | Alcohol |
| Sulfonate | Alkane |
Radical dehalogenation
Alkyl iodides and bromides are reduced with good yields to alkanes by radical dehalogenation with tri-n-butyltin hydride. Fluorides hardly react at all, whereas chlorides react with more difficulty. The order of reactivity for the halides is:
tertiary > secondary > primary
The reaction requires the presence of a radical initiator such as AIBN or peroxides. The reaction conditions are quite mild and compatible with a multitude of functions such as alkenes, carbonyl compounds, acids, lactones, etc.).
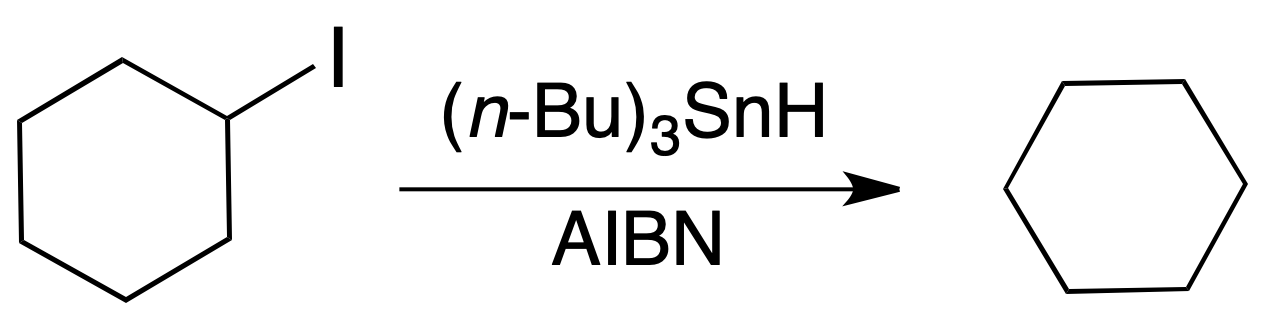
Its disadvantage is the toxicity of the reagent and the by-products generated.
Dehalogenation of vicinal halides
Because vicinal dihalides are obtained by addition of halogens to alkenes, this reaction is of relative interest.
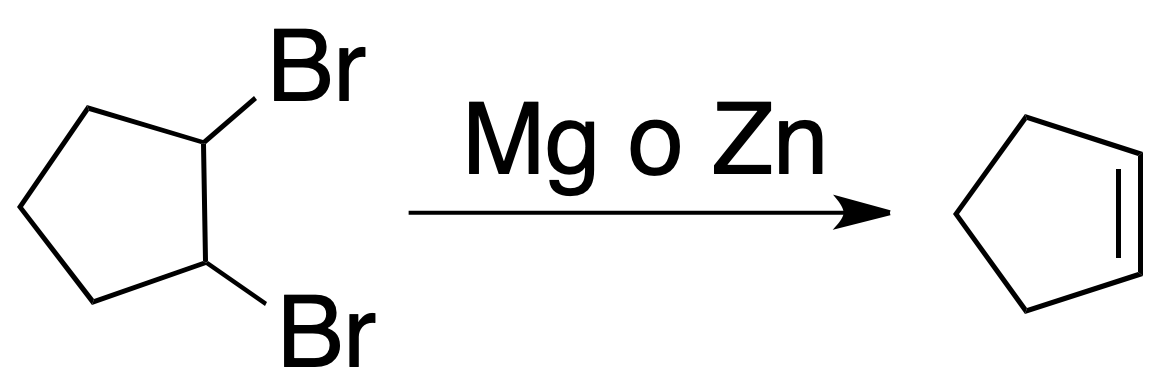
Wurtz synthesis (obtaining alkanes)
It consists of the formation of an alkane from two alkyl halides, by treatment with metallic sodium. In the latter case, the product mixtures are very significant.

Wurtz synthesis is one of the oldest organic reactions and is of historical interest today, due to its low yields and formation of by-products.
Corey-House synthesis (obtaining alkanes)
In the 1980s, Corey and House developed an alternative method to the Wurtz synthesis for the synthesis of alkanes, starting from an alkyl halide, which is reacted with Lio. The organolithium obtained is treated with CuI to give a lithium dialkylcuprate (Gilman‘s reagent), which is reacted with an alkyl halide.
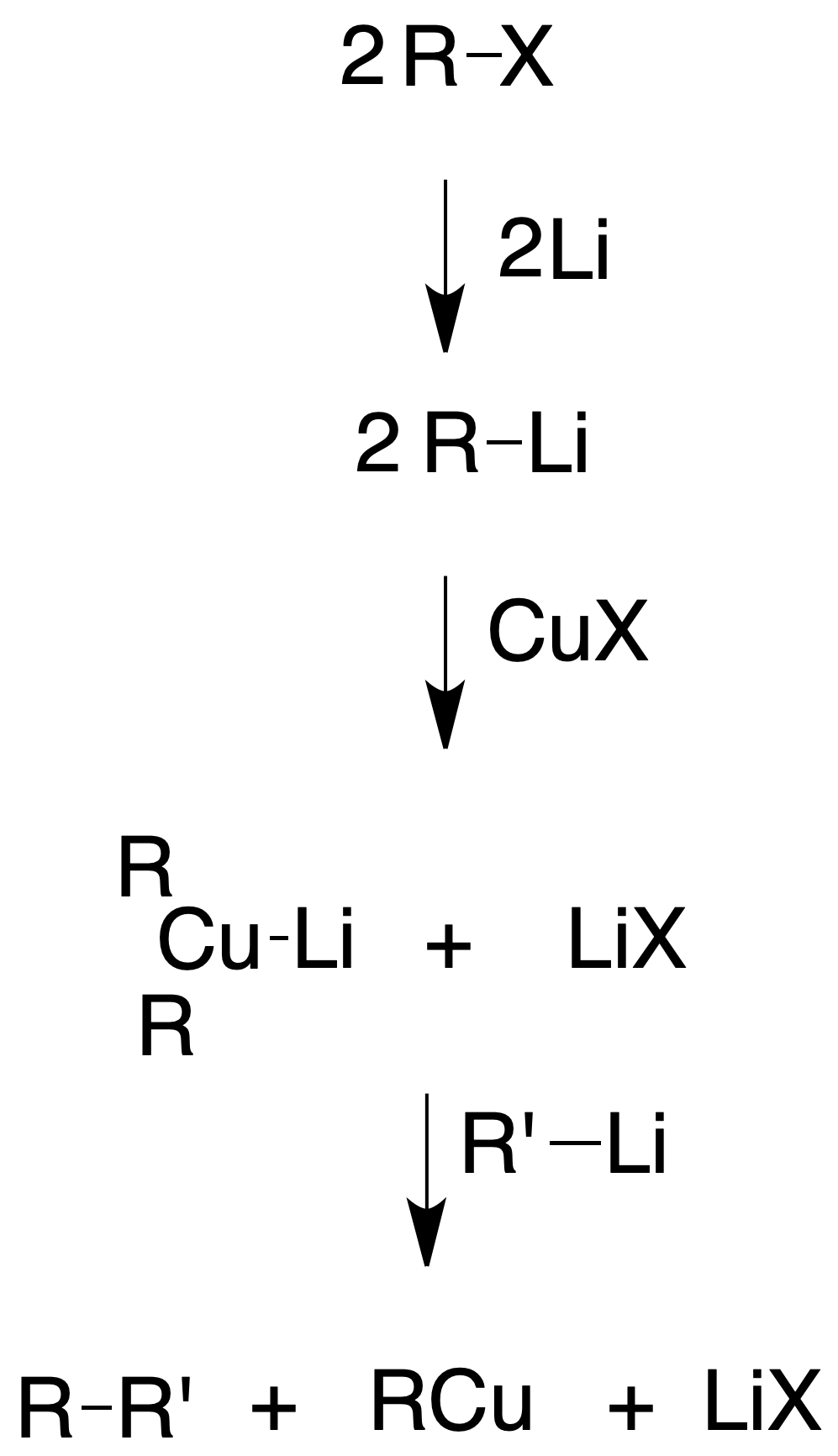
Unlike the Wurtz synthesis, much greater control is exercised over the products obtained, avoiding the formation of by-products and yields are substantially improved. The alkyl halide used in the last step cannot be tertiary, obtaining higher yields when it is primary or methyl.
Nucleophilic substitution
Elimination
Summary of substitution and elimination reactions
| Methyl / alkyl 1º | Alkyl 2º, 1º (or 2º stabilized by resonance) | Alkyl 3º | |
| SN2 | Strong nucleophiles | Never | |
| SN1 | Never | Weak nucleophiles | |
| E2 | Only when using strong non-nucleophilic bases | Any strong base | |
| E1 | Never | Weak base | |
| SN2 / E2 | Methyl halide does not give elimination. Halide 1º favors SN2 | Nucleophilic based mixtures | Only E2 |
| SN1 / E1 | Never | Mixtures even if H is not present at β | |
| Regiochemistry | Stereochemistry | |
| SN2 | – | Inversion of configuration |
| SN1 | Possibility of transpositions | Racemic mixtures |
| E2 | (Zaitsev’s rule) except with bulky bases. | Leaving group and antiperiplanar H. trans Alkene is formed if possible |
| E1 | (regla de Zaitsev) | Favored trans alkene |
Organometallic compounds
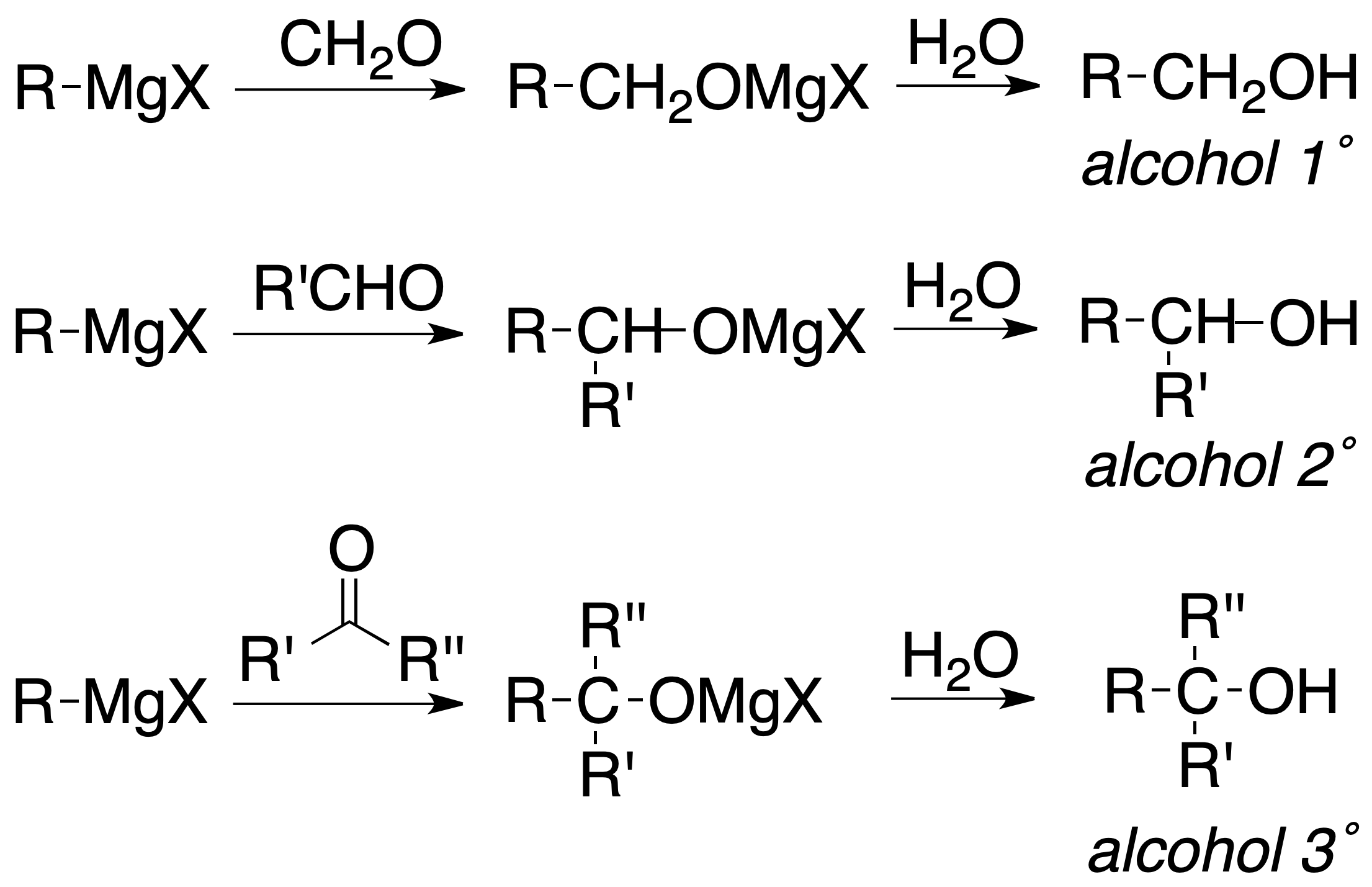
Alkyl, aryl and vinyl halides react with metals to give organometallic compounds. When the metal is Mg, the compound is called magnesian or Grignard reagent. Other metals used are Li, Cu or Zn. Organometallic compounds are good nucleophiles and have a strong basic character.
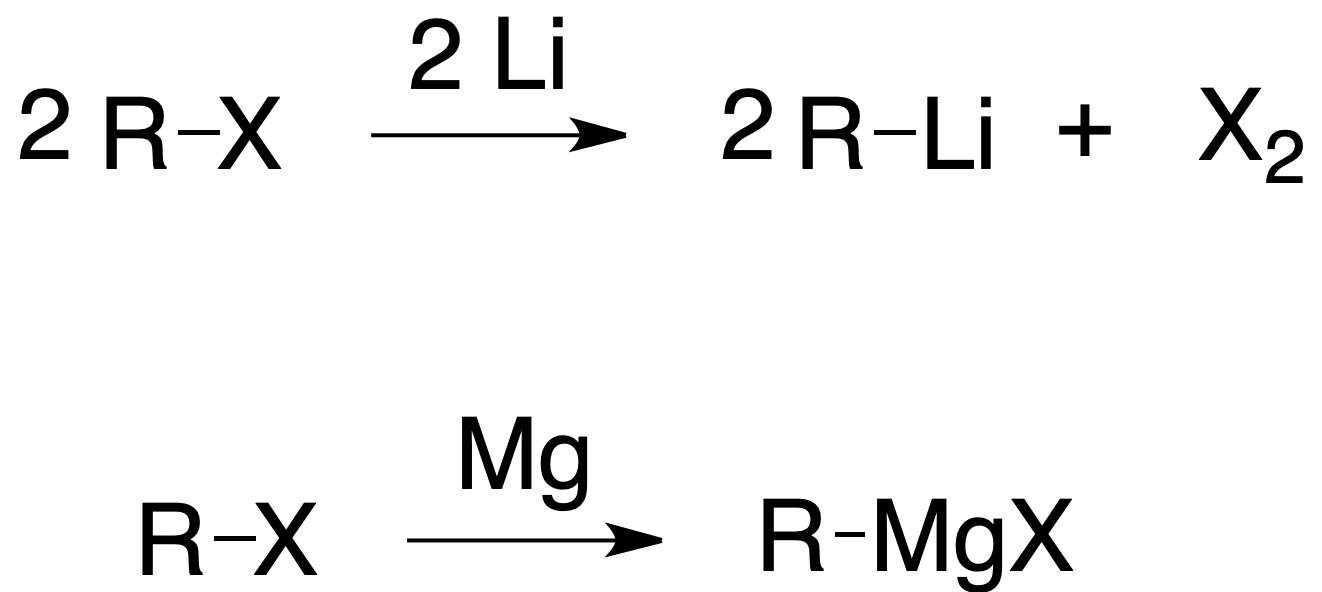
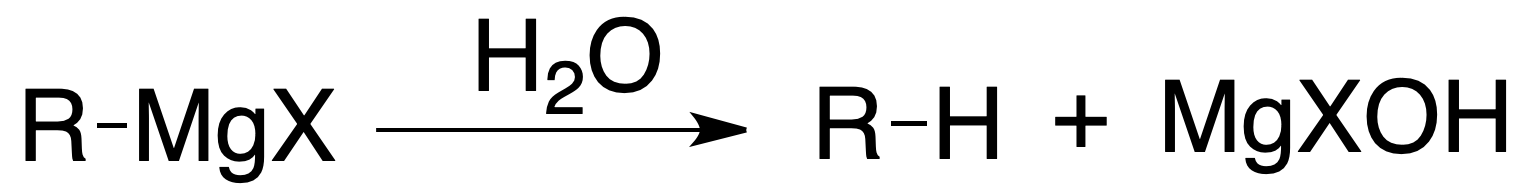
Grignard reagents undergo hydrolysis easily, being reduced to the corresponding hydrocarbon:
or added to carbonyl compounds to produce, after subsequent hydrolysis, alcohols: