What is Mannich reaction?
The Mannich reaction is a multicomponent condensation that was first reported in 1912. It involves an amine, an enolizable carbonyl compound (donor), and a nonenolizable carbonyl compound (acceptor) to form a β-amino carbonyl compound, known as the Mannich base. This reaction is commonly referred to as the Mannich reaction or Mannich condensation.
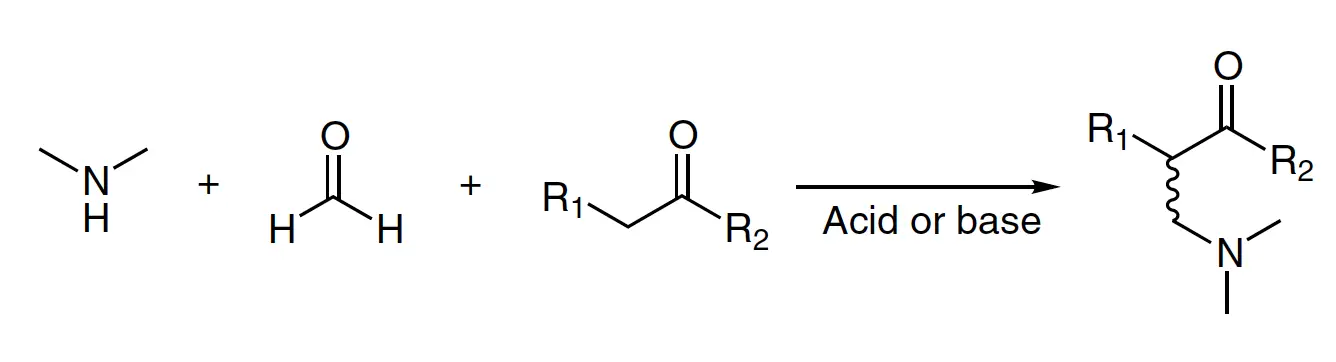
R1, R2 = H, alkyl, aryl (see list of acronyms)
A special case of the Mannich reaction occurs when the nonenolizable carbonyl compound is formaldehyde, which introduces a methylamino group onto the enolizable carbonyl compound. This is known as the Mannich aminomethylation.
The Mannich reaction has been extended to different variants, such as:
- aza-Mannich reaction
- halo-Mannich reaction
- azido-Mannich reaction
- vinylogous Mannich reaction
- nitro-Mannich reaction
- borono-Mannich reaction
- List-Barbas-Mannich reaction
The Mannich reaction can also be divided into direct and indirect Mannich reactions. In the direct Mannich reaction, aldehyde, unmodified ketone, and amines are used directly. In the indirect Mannich reaction, either the ketone is converted into a preformed enolate or its equivalent, or the acceptor carbonyl compound is transformed into an imine intermediate.
In addition to the enolate equivalent undergoing the Mannich reaction, electron-rich phenols also undergo the Mannich reaction with an imine component, whereas an electron-deficient phenol such as 2,6-dinitrophenol fails for this reaction.
The Mannich reaction is a highly popular reaction in modern organic synthesis due to its ability to yield desired compounds with high yields under simple conditions and with two potential chiral centers. Asides the already noted variants, the reaction has been extensively modified to include:
- aldimines coupled with silyl enol ether, silicon enolate, fluorovinyl ether, chiral aldimines with silyloxydienes, and fluorinated aldimines with aliphatic aldehydes
- iminium ions, such as cyclic iminium ions with enol derivatives (enol silane, vinyloxytrimethylsilane) and cyclic N-alkoxycarbonyl pyrrolinium ion with 2-trimethylsilyloxyfuran
- hydrozones, such as chiral N-acylhydrazones with silyl enolates, hydrazones with ketene silyl acetal, and hydrazone ester with silicon enolates
- imino esters, such as α-imino ester with aldehydes, N-PMP-protected α-imino glyoxylate with α,α-disubstituted aldehydes, α-imino ester with glycinated Schiff base, and N-acylimino ester with silyl enol ethers or vinyl ethers.
Other modifications include the coupling of acetate enolate equivalent with α-amido sulfones or PMP-imines, glyoxylate imines with ketene acetals, alkynyl imine with silylketene acetals, and phosphonylimine with hydroxyketones. Direct Mannich reactions have also been modified to occur between aldehydes, ketones, and carbamates, and between aldehydes, secondary amines, and methoxytris-(pentafluorophenyl)silane.
Similar to the aldol condensation, the Mannich reaction can be catalyzed by either acid or base, with different protic acids or Lewis acids, either alone or in combination with a different chiral ligand or auxiliary group, used to enhance the reaction’s stereoselectivity. Chiral basic molecules, such as pyrrolidines with substituents at the 2- and 4-positions (or 3- and 5-positions), are also good catalysts for the Mannich reaction.
The reaction has also been modified to occur under various conditions, such as microwave irradiation, phase-transfer reaction, in ionic liquid, in aqueous solution without organic co-solvent, VO(acac)2 catalyzed reaction via the generation of iminium ions in situ from amine oxides, and immobilization of chiral ligand onto the silica surface. The condensation between electron-rich phenol, amine, and a chiral α-N,N-dibenzylamino aldehyde has been reported to be temperature-sensitive, with high syn selectivity at high reaction temperatures and high anti-selectivity at low reaction temperatures. The Mannich reaction is a very useful reaction in organic synthesis.
References
Mannich, C. and Krösche, W. (1912), Ueber ein Kondensationsprodukt aus Formaldehyd, Ammoniak und Antipyrin. [On a condensation product of formaldehyde, ammonia and antipyrine.] Arch. Pharm. Pharm. Med. Chem., 250: 647-667. https://doi.org/10.1002/ardp.19122500151