Objective
The purpose of this experiment is to learn a series of basic techniques in an organic chemistry laboratory, such as:
- The use of reflux as a general technique to perform a hot reaction at the boiling temperature of a given solvent.
- Simple distillation as a procedure for the purification of liquid substances.
- The use of fractional distillation (or with rectification) for the purification of mixtures of liquid substances with e.g. proximate.
To achieve this objective, the synthesis of a simple ester such as ethyl acetate has been chosen.
Background
The esters of carboxylic acids can be formed by reaction between a carboxylic acid and an alcohol in the presence of a protic acid as catalyst, such as HCl or H2SO4 (called Fischer esterification, Nobel Prize in 1902) or a Lewis acid catalyst such as boron trifluoride,[1] or by reaction of an acid derivative such as acid chloride or anhydride with an alcohol.
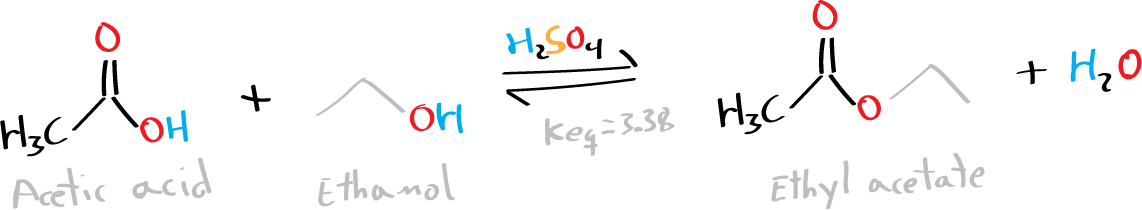
Esters are versatile compounds in organic chemistry and are widely used because they are easily converted to a wide variety of other functional groups.
Experimental procedure
In a flask of ground-glass mouth of 250 ml 40 ml of glacial acetic acid and 45 ml of ethanol EtOH of 96 % are placed. 5 ml of H2SO4 (conc.) are added dropwise with magnetic stirring. Then a reflux condenser and a drying tube are adapted to the flask. Once the assembly has been carried out, the reaction is heated at reflux for 1 h. After refluxing, the flask contents are allowed to cool.
The assembly is modified arranging it for a simple distillation in order to separate the ethyl acetate formed next to the ethanol EtOH that has not reacted and some acetic acid that is dragged. The distillation is maintained until no more liquid distills, remaining in the flask the H2SO4 and the acetic acid that has not reacted.
The distillate obtained is placed in a separatory funnel and shaken with a 10% sodium carbonate solution Na2CO3, taking care to open the tap immediately when mixing the two liquids, to allow the CO2 produced by the neutralization of the acetic acid to escape.[2] The ethyl acetate continues to be washed until no acid reaction is observed.[3]
The organic layer (upper) is separated and washed with a solution consisting of 20 g CaCl2 in 25 ml of water.[4] Again, it is decanted in two layers, discarding the aqueous layer (lower). The organic layer is transferred to a dry Erlenmeyer to which anhydrous Na2SO4 is added, to eliminate the water that it can contain. The mixture is shaken until a transparent solution is obtained.
After drying the ethyl acetate, the solid (Na2SO4) is separated by gravity filtration and the liquid obtained is purified by fractional distillation. The distillate obtained between 75 and 78 ºC is measured with a measuring cylinder and the amount by weight obtained is calculated, knowing that the density is 0.9 g·ml-1.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Ethyl acetate | 88.11 | -84 | 77.1 | 0.902 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic acid |   |
| EtOH |  |
| Ethyl acetate |   |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Ethyl acetate | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
References and notes
- [1] Usually forming a complex with diethyl ether.
- [2] The organic phase remains in the upper part of the separating funnel since ethyl acetate is less dense than water.
- [3] Check the pH of the aqueous solution with a piece of indicator paper. This should be basic, a sign that there is no acid left in the organic phase.
- [4] Calcium forms a water-soluble complex with EtOH.
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Vogel, A.I., Furniss, B.S., Hannaford, A.J., Tatchell, A.R., and Smith, P.W.G. (1989). Vogel’s Textbook of Practical Organic Chemistry (Vogel’s Textbook series). Longman. ISBN: 9780470214145