In esters analysis, most esters are low melting point liquids or solids, many with characteristic floral and fruity odors.
Their IR spectra show strong carbonyl bands from 1780 to 1720 cm-1 accompanied by two strong C-O absorptions in the region of 1300 to 1050 cm-1.
The main method for the characterization of an ester involves the identification of the alcohol and acid of which it is composed.
Hydrolysis of esters
The esters can be saponified by boiling with aqueous or alcoholic alkaline solutions, with concentrated HCl or with 40% H2SO4.
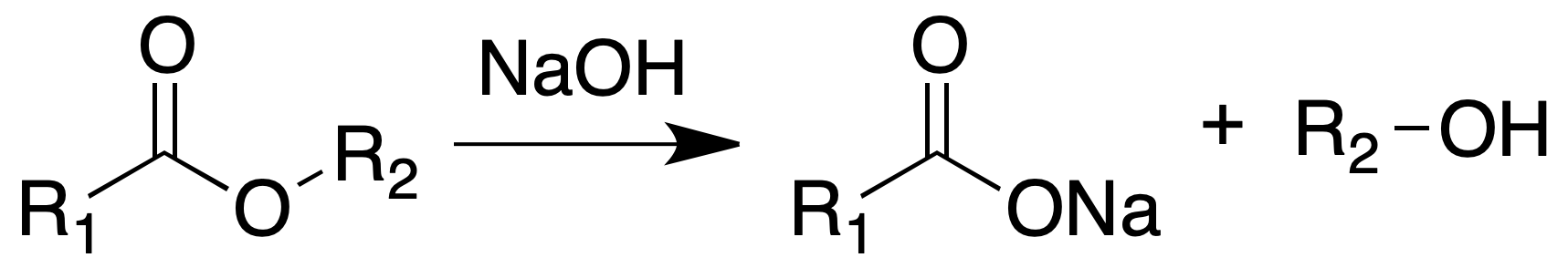
Procedure
This ester analysis is carried out in a small flask, where 200 mg to 1 g of ester are placed. Then add 2 to 10 ml of 25 % aqueous NaOH and proceed with magnetic stirring.
Mount a condenser and heat at reflux for 30 min (if the ester boils below 110 ºC) or for 2 h (if it boils above that temperature).
Cool and acidify with dilute acid (phosphoric acid is recommended). Recover the acid by filtration or extraction.
Conversion into amides
Many esters when heated with concentrated ammonia solutions are transformed into amides.
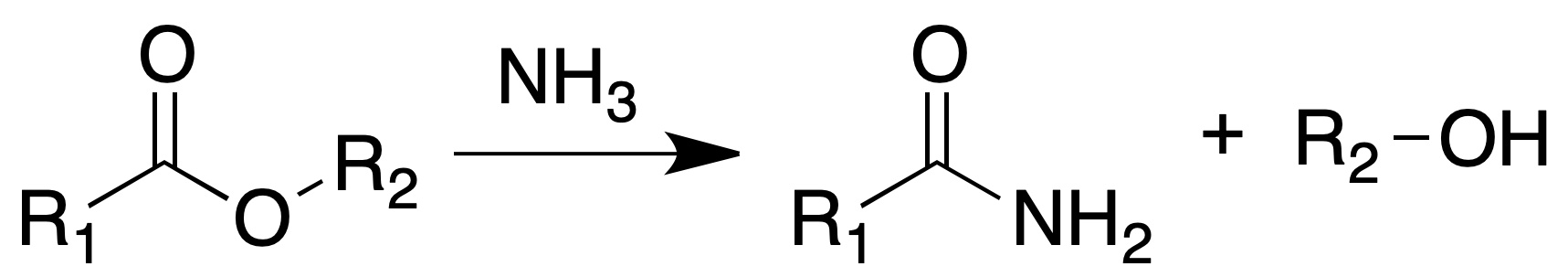
Likewise, when heated for several hours with hydrazine hydrate, they are converted into hydrazides.