Objective
To prepare the high valued-added methyl salicylate from commercial aspirin tablets by two simultaneous transesterification-esterification reactions.
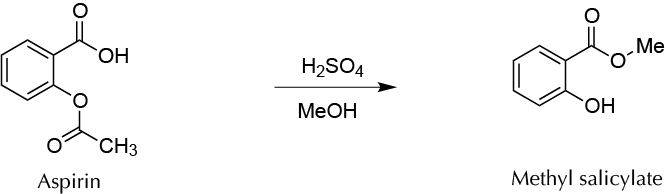
Background
Methyl salicylate is an ester salicylic acid derivative found in many plants. Wintergreen Gaultheria procumbens is one of the species with the highest concentration of this compound, and therefore this molecule is known as Gaultheria essential oil. It can be used as an additive in food, because it has a flavor similar to mint. Gaultheria essential oil is used as an antiseptic for preparing creams and liniments against muscle pain and bruises. In this experiment, this compound is synthesized using commercial aspirin tablets as the starting material. Once the excipients and additives of the tablets are eliminated, the remaining acetyl salicylic acid is converted into methyl salicylate by two simultaneous processes of transesterification–Fischer-Speier esterification catalyzed by acid. Methyl salicylate and the volatile methyl acetate are formed at the same time, the latter being removed by evaporation when reaction crude is processed.
Microscale experimental procedure
Weigh three aspirin tablets and grind them with a mortar and pestle. Transfer the powder to a 5 ml conical vial, and add 3.5 ml of MeOH. Fit a stopper and shake vigorously for 2 min. Filter the mixture with a Pasteur pipette with a piece of cotton tip to eliminate all the additives and excipients. Transfer the clear solution to a clean conical vial with a spin vane, and add 300 μl of H2SO4 (conc.). Fit a water condenser with a drying tube, and reflux the mixture for 90 min. Afterward, cool the mixture to r.t.
Transfer the reaction mixture to a suitable separatory funnel, containing 5 ml of satured NaHCO3 solution. Gently shake the mixture by hand without a stopper to avoid CO2 overpressure (check that all the acid has been eliminated with pH test paper). Extract the solution with CH2Cl2 (2 × 5 ml). Join the organic layer in a clean 25 ml Erlenmeyer, and dry the organic solution with anhydrous Na2SO4. Filter the mixture to eliminate the desiccant on a tared round-bottom flask, and eliminate the solvent under vacuum (rotary evaporator) to give a liquid corresponding to methyl salicylate. Weigh the flask and determine the yield.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetylsalicylic acid | 180.16 | 134-136 | - | - |
| CO2 | 44.01 | -78.5 | - | - |
| CH2Cl2 | 84.93 | -97 | 40.0 | 1.33 |
| H2SO4 | 98.08 | 3 | - | 1.80-1.84 |
| MeOH | 32.04 | -98 | 64.7 | 0.791 |
| Methyl salicylate | 152.15 | (-)8-(-)7 | 222 | 1.174 |
| Na2SO4 | 142.04 | 884 | - | 2.630 |
| NaHCO3 | 84.01 | 300 | - | 2.160 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetylsalicylic acid |  |
| CO2 |  |
| CH2Cl2 |  |
| H2SO4 |  |
| MeOH |    |
| Methyl salicylate |  |
| Na2SO4 | Non-hazardous |
| NaHCO3 | Non-hazardous |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetylsalicylic acid | BSYNRYMUTXBXSQ-UHFFFAOYSA-N |
| CO2 | CURLTUGMZLYLDI-UHFFFAOYSA-N |
| CH2Cl2 | YMWUJEATGCHHMB-UHFFFAOYSA-N |
| H2SO4 | QAOWNCQODCNURD-UHFFFAOYSA-N |
| MeOH | OKKJLVBELUTLKV-UHFFFAOYSA-N |
| Methyl salicylate | OSWPMRLSEDHDFF-UHFFFAOYSA-N |
| Na2SO4 | PMZURENOXWZQFD-UHFFFAOYSA-L |
| NaHCO3 | UIIMBOGNXHQVGW-UHFFFAOYSA-M |
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- A. M. Hartel and J. M. Hanna, Preparation of oil of wintergreen from commercial aspirin tablets. A microscale experiment highlighting acyl substitutions, Journal of Chemical Education 86 (2009), no. 4, 475, DOI: 10.1021/ed086p475