Written by J.A Dobado | Last Updated on April 22, 2024
The analysis of aromatic hydrocarbons gives negative results in the tests with bromine in CCl4 and with alkaline permanganate, and they also burn with a characteristic dark flame.
Most alkylated aromatic hydrocarbons are liquids, but those with more than one ring (fused or not) are solids.
- Solubility in H2SO4: Simple aromatic hydrocarbons are insoluble in H2SO4 but soluble in fuming H2SO4. When two or more alkyl substituents are present such hydrocarbons are easily sulfonated by dissolving in concentrated H2SO4. Condensed aromatic hydrocarbons, such as naphthalene and anthracene, react slowly with concentrated H2SO4.
- IR spectrum: They show the characteristic absorption bands of the aromatic ring.
Nitration test
The analysis of aromatic hydrocarbons with the nitration test generally produces an increase in temperature by cold treatment with a concentrated H2SO4/HNO3 mixture. We can use this reaction at the preparative level.
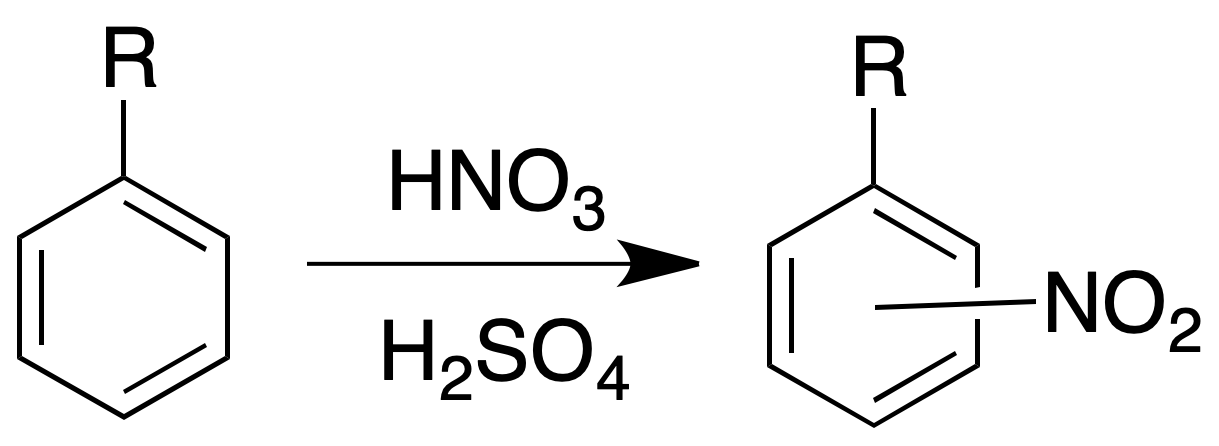
Procedure: We add 500 mg of product to about 3 ml of concentrated H2SO4. It is added equal volume of concentrated HNO3 drop by drop on the previous dissolution, shaking after each addition. It is heated for 5 to 10 min in a water bath at 60 ºC.
Remove the tube from the bath and stir frequently. Cool and pour over ice water. Filter the precipitate, wash it with water and recrystallize it in EtOH/H2O.
Formation of arylbenzoic acid
Procedure: In a flask with reflux place 400 mg of phthalic anhydride, 10 ml of carbon sulfide, 800 mg of anhydrous aluminum chloride and 400 mg of the aromatic hydrocarbon. Heat the mixture for about 30 min in a water bath and then cool on tap.
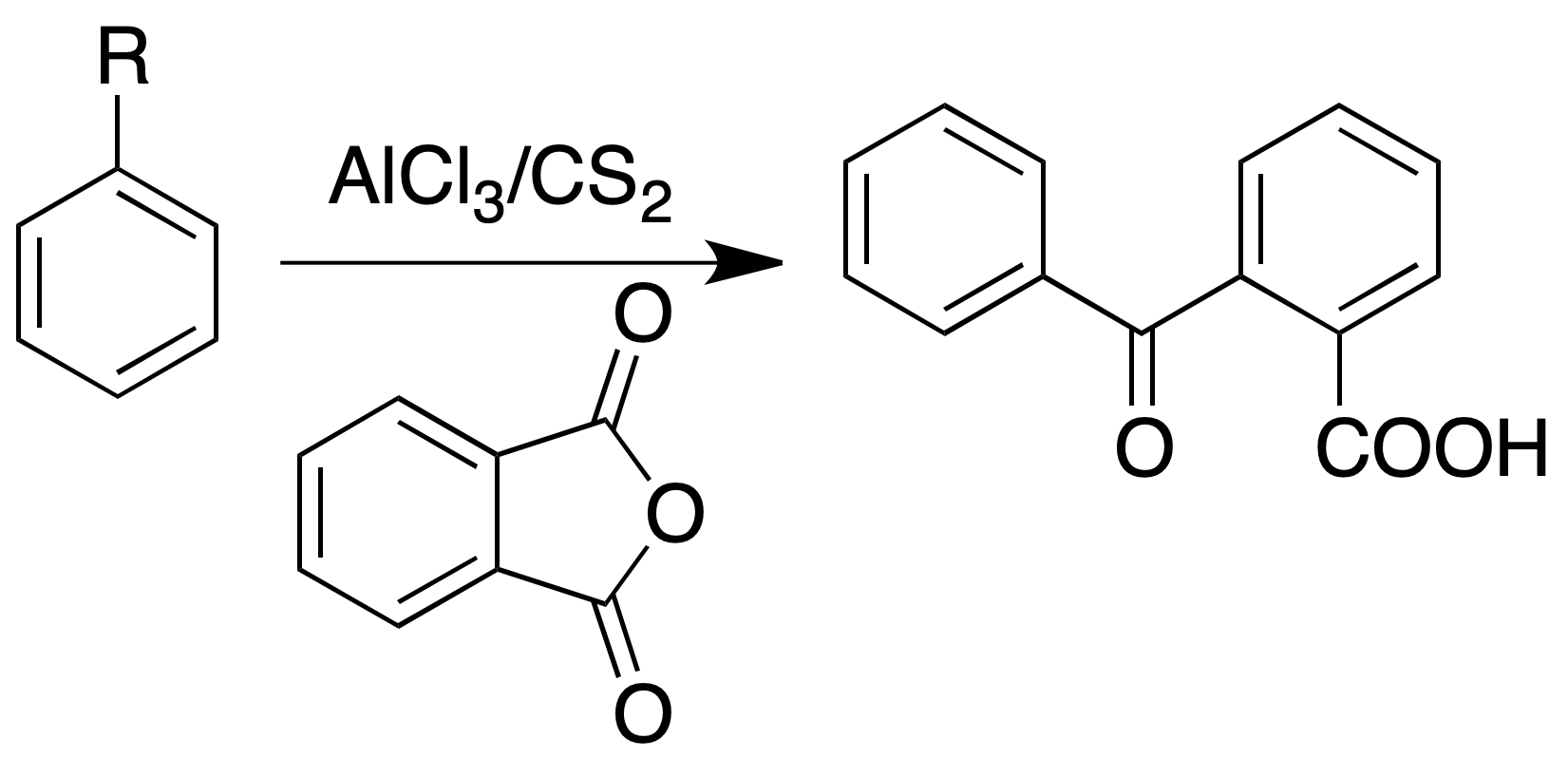
Decant the carbon sulfide layer slowly. To add cooling 5 ml of concentrated HCl and then 5 ml of water. Shake the mixture vigorously. Collect the solid and wash it with cold water. If it does not crystallize, the oil is extracted with dilute ammonium hydroxide and then neutralized with HCl (conc).