Almost all amides are colorless crystalline solids. In the analysis of amides, the observation of the infrared (IR) spectrum is important. In this spectrum, two bands are observed in the region of the carbonyl group (amide I and II) in addition to the stress band of the N-H group in the region of 3400 cm-1.
It is not always easy to classify them, so they can be hydrolyzed using the following chemical tests:
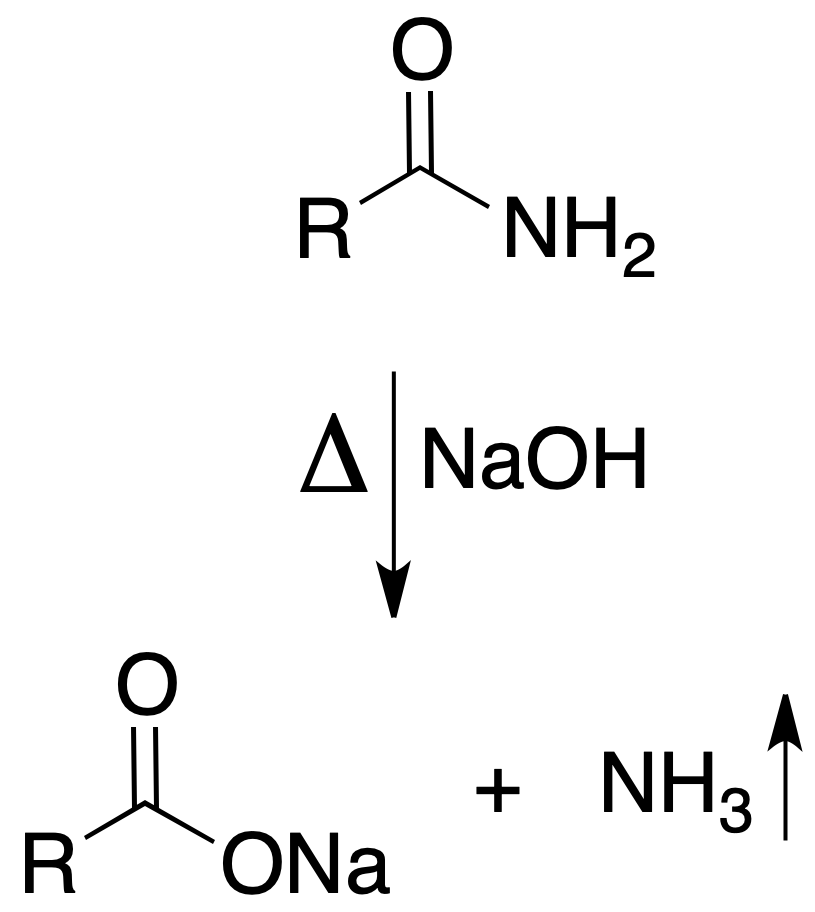
Sodium hydroxide test
By boiling a mixture of 200 mg of primary amide with 5 ml of 10 % NaOH, ammonia, recognizable by its odor and/or with indicator paper, is released.
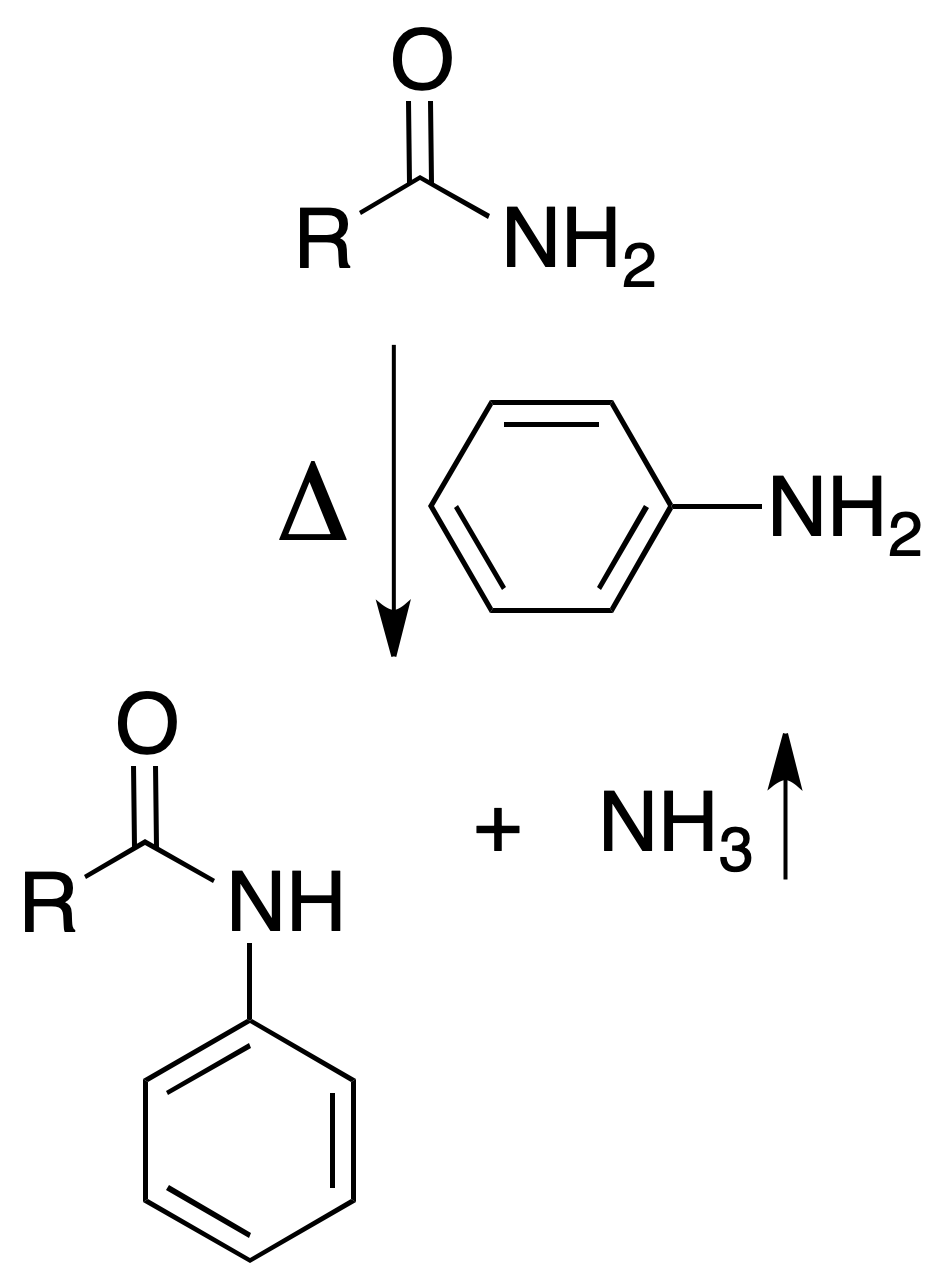
When we heat primary amides with aniline they give off ammonia with formation of anilides.
Mercury oxide test
The last reaction described for amide analysis is the mercuric oxide test. Here, some amides react with mercury oxides to form mercuric salts.