Objective
The purpose of the experiment is to become familiar with the methods of synthesis of dyes of the phthaleins type. The synthesis is carried out by condensation of phthalic anhydride with phenol, and involves an (acid-catalyzed) reaction of electrophilic aromatic substitution SEAr.
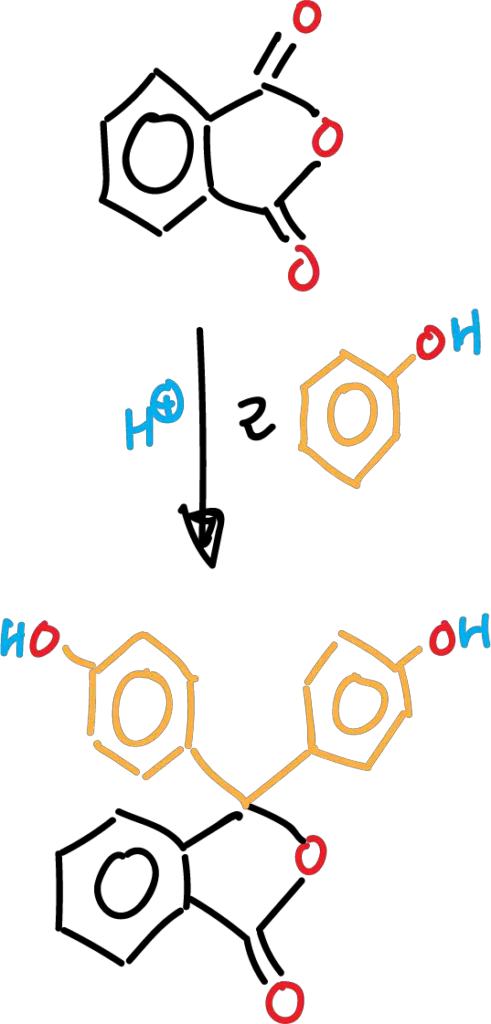
The phenolphthalein is purified by recrystallization of the final product. Once recrystallized, the behavior of phenolphthalein, as an indicator, will be observed in acidic and basic media.
Background
Phenolphthalein (C20H14O4) is a pH indicator. In acidic solutions it remains colorless, but in the presence of basic solutions it takes a pinkish color with a turning point between pH = 8.0 (colorless) to pH = 9.8 (magenta or pinkish).
However, at extreme pH (very acidic or basic) it shows other changes in coloration: at strongly basic pH it becomes colorless, while at strongly acidic pH it changes to orange.
This color change is due to the conjugation of the π electrons of the phenolphthalein derivative which at different pH absorbs at visible light wavelengths. It is related to the different open and closed structure of the lactonic ring present in these compounds.
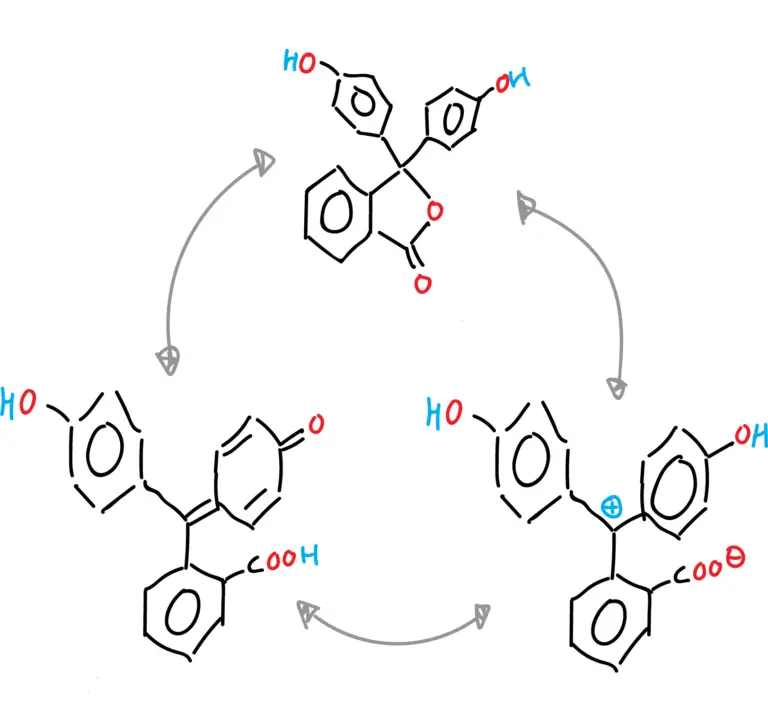
The different forms of phenolphthalein are summarized in the following figures:
- Cationic form of phenolphthalein.
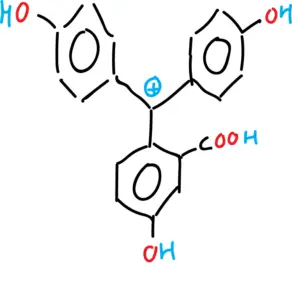
- Anionic form of phenolphthalein (monoanion and dianion).
- Neutral form of phenolphthalein (tautomeries: lactonic, zwitterionic and quinonic).
Phenolphthalein is insoluble in water and is usually dissolved in alcohols for use. It is a weak acid that can lose H⊕ protons in solution. Therefore, the main applications of phenolphthalein derivatives are as dyes and pH indicators.
Phthalein derivatives can be synthesized from the reaction between two moles of the phenolic derivative with one mole of the anhydride derivative in the presence of different catalysts, among which we can mention: AlCl3, SnCl4, ZnCl2 and H2SO4, among others.
Reaction mechanism
The reaction is catalyzed by acid (methanesulfonic acid, MeSO4H). The reaction mechanism starts, in a first step, with the protonation of the phthalic anhydride as shown in figure, giving rise to the corresponding carbocation which is in a tautomeric equilibrium.
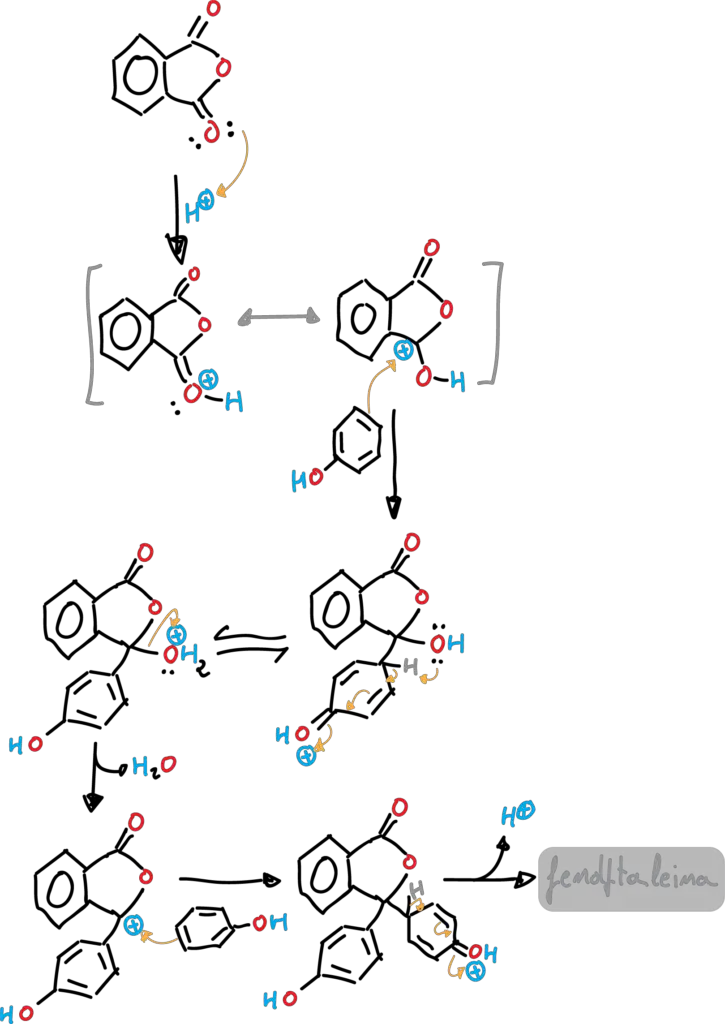
In a second step, a π-bond of the phenol attacks the carbocation that has formed. The corresponding Wheland intermediate of reaction SEAr is produced. Next, the unshared electron pair of an oxygen attacks the hydrogen in the para position of the initial phenol. Subsequently, a water molecule is released, which generates another carbocation. The reaction continues, with another SEAr attack of another phenol molecule, and ends with the loss of a proton to give phenolphthalein.
Experimental procedure
0.1552 g (1 mmol) of phthalic anhydride, 0.1881 g (2 mmol) of phenol and methanesulfonic acid, MeSO4H, 3 ml (0.046 mmol) are placed in a 100 ml round bottom flask. A reflux cooler, with magnetic stirring, is attached and the flask is placed in an oil bath. The bath is heated for 2 hours at 90.5 °C, and the reaction is monitored by TLC every 30 minutes.
Once the process is complete, the reaction is terminated by adding 2 mL of methanol, MeOH, to the round bottom flask and cooling slightly, before allowing it to cool, subsequently, further in an ice water bath for 5 minutes, to allow crystallization of the phenolphthalein.
Once the precipitate is formed, it is filtered to vacuum and the phenolphthalein crystals are collected. The product is recrystallized from methanol and tan-brown crystals are obtained. The yield of the dry product is calculated. The estimated yield is 73-94 %, and the melting point 259-261 ºC.
Phenolphthalein identification reaction
Dissolve some crystals of phenolphthalein in 1 mL of ethanol, add 1 mL of distilled water, shake and add a few drops of soda solution. Note your observations.
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| HCl | 36.46 | -30 | >100 | 1.200 |
| Methanesulfonic acid | 96.10 | 17-19 | 169 | 1.480 |
| Phthalic anhydride | 148.10 | 131.6 | 295 | 1.530 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| Phenol | 94.11 | 40-42 | 182 | 1.07 |
| Phenolphthalein | 318.33 | 258-263 | 557.8 | 1.277 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| MeOH | 32.04 | -98 | 64.7 | 0.791 |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| HCl |   |
| Methanesulfonic acid |  |
| Phthalic anhydride |    |
| EtOH |  |
| Phenol |    |
| Phenolphthalein |  |
| NaOH |  |
| MeOH |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| Methanesulfonic acid | AFVFQIVMOAPDHO-UHFFFAOYSA-N |
| Phthalic anhydride | LGRFSURHDFAFJT-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| Phenol | ISWSIDIOOBJBQZ-UHFFFAOYSA-N |
| Phenolphthalein | KJFMBFZCATUALV-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| MeOH | OKKJLVBELUTLKV-UHFFFAOYSA-N |
Video on phenolphthalein synthesis
References
- Isac-García, J.; Dobado, J. A.; Calvo-Flores, F. G.; and Martínez-García, H. (2015). Experimental Organic Chemistry Laboratory Manual. Elsevier Science & Technology. ISBN: 978-0-12-803893-2
- Reactions of phenolphthalein at various pH values
Georg Wittke
Journal of Chemical Education 1983 60 (3), 239
DOI: 10.1021/ed060p239 - Phenolphthalein and methyl orange
Charles A. Peters and Bryan C. Redmon
Journal of Chemical Education 1940 17 (11), 525
DOI: 10.1021/ed017p525