Objective
To prepare and isolate two isomers in the nitration reaction of 4-methylaniline using two synthetic strategies.
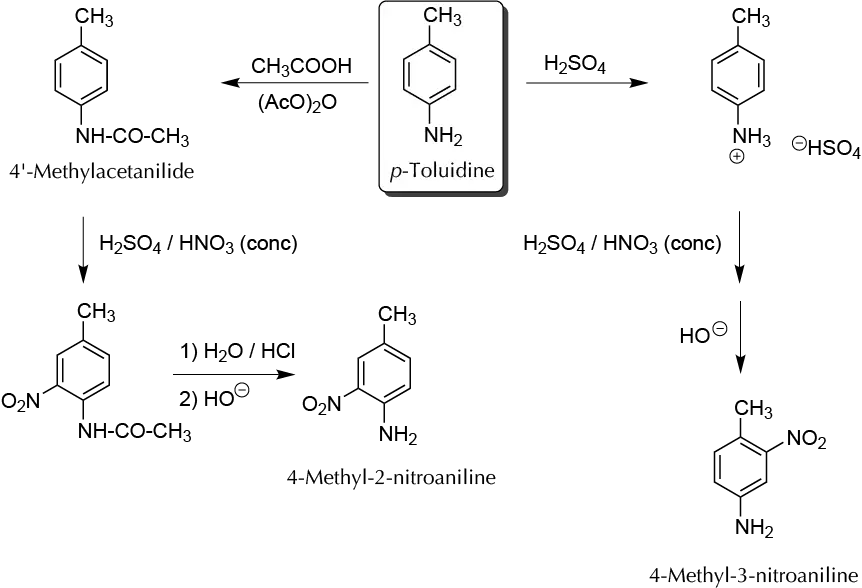
Background
In electrophilic aromatic substitution reactions, the amino groups have a strong activating character directing ortho and para. However, when the amino group is protonated due to the positive charge on the nitrogen atom, it is strongly deactivating and directs meta. Therefore, to perform a nitration reaction at ortho with respect to the -NH2 group, it is necessary first to form the acetylated derivative of the amine, and to release the amino group (deprotection reaction) after the nitration step. This is because the reagent used for nitrating is a mixture of H2SO4 and HNO3, signifying that the group -NH2 should be as -NH3⊕. On the other hand, the possibility of oxidation of an amino group is minimized.
Microscale experimental procedure
A) Preparation of 4-methyl-3-nitroaniline:
Dissolve, in a 2 ml conical vial at r.t. with magnetic stirring, 107 mg of p-toluidine in 200 μl of concentrated H2SO4. Cool the solution in an ice bath and continue stirring while adding dropwise 400 μl of a solution (prepare the solution mixing 300 μl of concentrated HNO3 and 100 μl of concentrated H2SO4). The addition must made while maintaining the temperature of the reaction mixture below 5 ºC. After the addition, allow the reaction to reach r.t. and continue stirring for 15 min. Then pour the reaction crude into a 5 ml NaOH 40% solution and stir until a yellow precipitate appears. Isolate the solid by vacuum filtration using a Hirsch funnel and wash it with 10 ml of cold deionized water. Purify by recrystallization from 95% EtOH in a Craig tube (do not use more than 3 ml of solvent). The estimated yield is 75% (m.p. 78–79 ºC).
B) Acylation of p-toluidine:
In a conical vial with a spin vane, prepare a solution of 107 mg (1 mmol) of p-toluidine in 500 μl acetic acid. Then add 200 μl of acetic anhydride. Fit a water condenser and reflux the mixture with magnetic stirring for 15 min. Upon completion of the reflux, cool the reaction crude to r.t. and pour it into 5 ml of ice water. Filter the solid under vacuum using a Hirsch funnel and wash it with 10 ml of deionized water. Dry and weigh the solid and calculate the yield (estimated yield 75%). Use this product for the following steps, adjusting in each case the amounts of reagents.
C) Nitration of 4-methylacetanilide:
In a conical vial, place 100 mg (0.67 mmol) of 4-methylacetanilide together with a mixture of 300 μl of concentrated HNO3 and 100 μl of concentrated H2SO4. Fit a water condenser to the vial and heat the reaction mixture to 50 ◦C for 15 min with magnetic stirring. After this time, allow the reaction crude to cool to r.t., and pour it into a 5 ml Erlenmeyer containing an ice-water mixture. Vacuum filter the yellow solid that precipitates, and wash the crystals with 10 ml of cold water. Dry under an air steam, weigh, and calculate the yield (estimated 70%; m.p. = 91–93 ºC).
D) Hydrolysis of 4-methyl-2-nitroacetanilide:
Prepare, in a conical vial with a spin vane, a suspension of 80 mg (0.41 mmol) of 4-methyl-2-nitroacetanilide in 500 μl of concentrated HCl. Couple a water condenser and reflux the mixture for 30 min. After the reflux period, cool to r.t., and add dropwise a solution of NaOH 0.5 M until reaching a basic pH. A solid precipitates; filter under vacuum and recrystallize the product from EtOH in a Craig tube (estimated yield 70%; m.p. = 116–117 ºC).
Physico-chemical properties
This table collects data for the molecular weight (Mw), melting point (M.p.) boiling point (B.p.) and density of the reactives and compounds used in this laboratory experiment.
| Name | Mw (g/mol) | M.p. (ºC) | B.p. (ºC) | Density (g/ml) |
| Acetic acid | 60.05 | 16.2 | 118 | 1.049 |
| Acetic anhydride | 102.09 | -73.1 | 139.8 | 1.080 |
| EtOH | 46.07 | -114.1 | 78.5 | 0.790 |
| HCl | 36.46 | -30 | >100 | 1.200 |
| NaOH | 40.00 | 318 | 1,390 | 2.130 |
| p-Toluidine | 107.15 | 41-46 | 200 | - |
| 4'-Methylacetanilide | 149.19 | 149-151 | 307 | - |
| 4-Methyl-2-nitroaniline | 152.15 | 115-116 | - | - |
| 4-Methyl-3-nitroaniline | 152.15 | 74-77 | - | - |
GHS pictograms
Hazard pictograms form part of the international Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and are collected in the followinf Table for the chemical compounds used in this experiment.
| Name | GHS |
| Acetic acid |   |
| Acetic anhydride |    |
| EtOH |  |
| HCl |   |
| NaOH |  |
| p-Toluidine |    |
| 4'-Methylacetanilide |  |
| 4-Methyl-2-nitroaniline |    |
| 4-Methyl-3-nitroaniline |    |
International Chemical Identifier
The IUPAC InChI key identifiers for the main compounds used in this experiment are provided to facilitate the nomenclature and formulation of chemical compounds and the search for information on the Internet for these compounds.
| Acetic acid | QTBSBXVTEAMEQO-UHFFFAOYSA-N |
| Acetic anhydride | WFDIJRYMOXRFFG-UHFFFAOYSA-N |
| EtOH | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
| HCl | VEXZGXHMUGYJMC-UHFFFAOYSA-N |
| NaOH | HEMHJVSKTPXQMS-UHFFFAOYSA-M |
| p-Toluidine | RZXMPPFPUUCRFN-UHFFFAOYSA-N |
| 4'-Methylacetanilide | YICAMJWHIUMFDI-UHFFFAOYSA-N |
| 4-Methyl-2-nitroaniline | DLURHXYXQYMPLT-UHFFFAOYSA-N |
| 4-Methyl-3-nitroaniline | GDIIPKWHAQGCJF-UHFFFAOYSA-N |